Functional analysis and strain optimization of industrially relevant Bacilli
Bacillus subtilis is one of the best-studied bacteria and is known for its ability to form metabolically inactive spores to survive under extreme environmental conditions. In addition, B. subtilis is a master at forming other differentiated cell types that play important roles in motility, biofilm formation or the uptake of free DNA from the environment. In addition to this cell differentiation, bacilli are able to secrete large amounts of biotechnologically relevant substances, which is why representatives such as B. subtilis or B. licheniformis have become crucial for the industrial production of vitamins, enzymes or secondary metabolites.
In close collaboration with our academic and industrial partners, we analyze the physiology of different Bacilli and modify these cell factories. We use a variety of methods to gain insight into the growth and production behavior, expression and regulation of selected genes, or to visualize specific differentiation processes of individual cells. State-of-the-art molecular techniques for cloning and genome editing (e.g. CRISPR/Cas or Cre/lox) also allow us to create new strain variants and optimize existing Bacillus expression platforms.
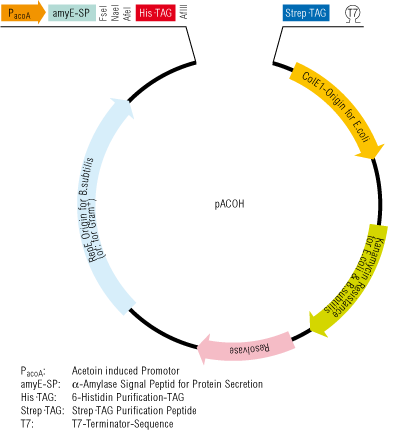
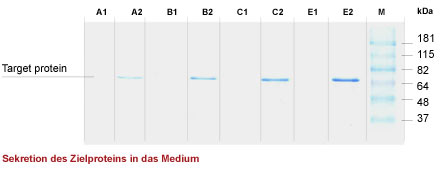
Fig. 1. Schematic presentation of an acetoin-regulated Bacillus subtilis expression system and an example of the extracellular protein production with this system
Responsible staff members:
Dr. Britta Jürgen, Dr. Norma Welsch, Maximilian Hilkmann, Jana Matulla
Literature:
- Trung NT, Hung NM, Thuan NH, Canh NX, Schweder T, Jürgen B. 2019. An auto-inducible phosphate-controlled expression system of Bacillus licheniformis. BMC Biotechnol. 19(1):3.
- Handtke S, Albrecht D, Zühlke D, Otto A, Becher D, Schweder T, Riedel K, Hecker M, Voigt B. 2017. Bacillus pumilus KatX2 confers enhanced hydrogen peroxide resistance to a Bacillus subtilis PkatA::katX2 mutant strain. Microb Cell Fact. 16(1):72.
- Kumpfmüller J, Methling K, Fang L, Pfeifer BA, Lalk M, Schweder T. 2015. Production of the polyketide 6-deoxyerythronolide B in the heterologous host Bacillus subtilis. Appl Microbiol Biotechnol. 100(3):1209-20.
- Zobel S, Kumpfmüller J, Süssmuth RD, Schweder T. 2014. Bacillus subtilis as heterologous host for the secretory production of the non-ribosomal cyclodepsipeptide enniatin. Appl Microbiol Biotechnol. 99(2):681-91.
- Voigt B, Schroeter R, Schweder T, Jürgen B, Albrecht D, van Dijl JM, Maurer KH, Hecker M. 2014. A proteomic view of cell physiology of the industrial workhorse Bacillus licheniformis. J Biotechnol. 191:139-49.
- Handtke S, Schroeter R, Jürgen B, Methling K, Schlüter R, Albrecht D, van Hijum SA, Bongaerts J, Maurer KH, Lalk M, Schweder T, Hecker M, Voigt B. 2014. Bacillus pumilus reveals a remarkably high resistance to hydrogen peroxide provoked oxidative stress. PLoS One. 9(1):e85625.
- Kumpfmüller J, Kabisch J, Schweder T. 2013. An optimized technique for rapid genome modifications of Bacillus subtilis. J Microbiol Methods. 95(3):350-2.
- Kabisch J, Pratzka I, Meyer H, Albrecht D, Lalk M, Ehrenreich A, Schweder T. 2013. Metabolic engineering of Bacillus subtilis for growth on overflow metabolites. Microb Cell Fact. 12:72.
- Kabisch J, Thürmer A, Hübel T, Popper L, Daniel R, Schweder T. 2013. Characterization and optimization of Bacillus subtilis ATCC 6051 as an expression host. J Biotechnol. 163(2):97-104.
- Enfors SO, Jahic M, Rozkov A, Xu B, Hecker M, Jürgen B, Krüger E, Schweder T, Hamer G, O'Beirne D, Noisommit-Rizzi N, Reuss M, Boone L, Hewitt C, McFarlane C, Nienow A, Kovacs T, Trägårdh C, Fuchs L, Revstedt J, Friberg PC, Hjertager B, Blomsten G, Skogman H, Hjort S, Hoeks F, Lin HY, Neubauer P, van der Lans R, Luyben K, Vrabel P, Manelius Å. 2001. Physiological responses to mixing in large scale bioreactors. J Biotechnol. 85(2):175-85.